So Much More Than ‘Just A Headache’
Unfamiliar with the extreme pain a migraine brings, it’s natural to try and relate it to a bad headache – mistaking the condition’s throbbing pain for a common distracting ache. Besides intense pain, migraines also come with a host of sensory issues such as severe sensitivity to sound and light (known as photophobia), neck stiffness, and visual phenomena. While headaches are an infamous symptom of illness, migraines are the illness.
The most striking difference between a migraine and a headache is that, almost as if they have a sixth sense, one in three migraine sufferers can predict when one is brewing before the pain sets in. Relax – we’re not omniscient. In the build up to a migraine attack, we experience auras: transient visual disturbances that are as disorientating as they are fascinating from a scientific perspective.

What starts out as a shimmering haze in the migraine sufferer’s periphery, gradually envelops their field of vision with flashing lights, shimmering arcs, and blind spots. Although auras serve as a warning for an impending migraine attack, they can be far more dangerous than the ensuing headache; during auras, brain cells can become damaged, even to the point of death, causing ischaemic strokes and creating lesions in the white matter of the brain. But what’s the connection between a migraine and these strange visual symptoms?
Origin of an Aura
At the onset of a migraine, the biochemistry of the brain changes drastically. At rest, healthy neurons contain fewer positively charged ions such as sodium ions (Na+) and potassium ions (K+) and more negatively charged proteins and nucleic acids than their surroundings. This separation of charge gives their membranes a low resting potential of -70 mV.
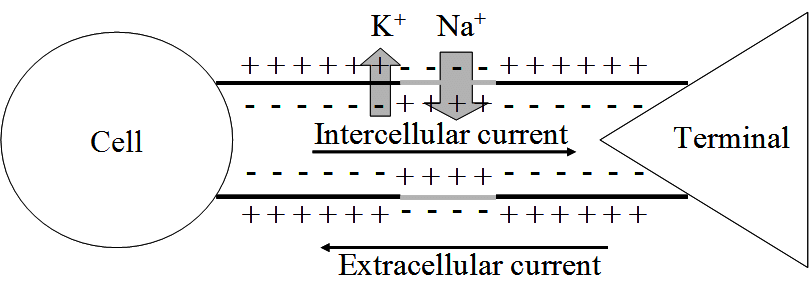
If we compare a neuron’s axon to a simple tube connecting one neuron to its neighbour, we can see that the inside of the axon is more negative than its surroundings outside the cell (the extracellular space).
Triggering the migraine aura is a sudden, spontaneous efflux of K+ ions out of a single neuron buried deep in the brain’s visual cortex – causing the cell’s membrane potential to plummet. The build-up of K+ ions in the extracellular space triggers the release of amino acid glutamate which excites neighbouring neurons and allows K+ ions to flood in – propagating the ion movement further. On the heels of this sudden flurry of activity, comes a wave of neuronal silence in which each cell stops processing and transferring information as it slowly returns to normal.
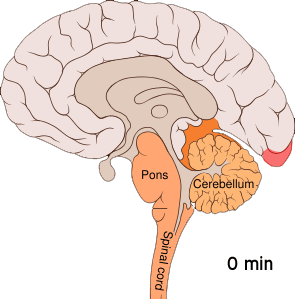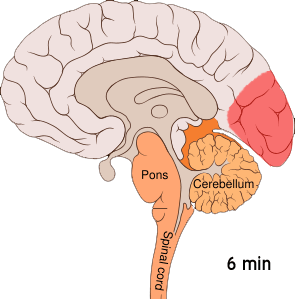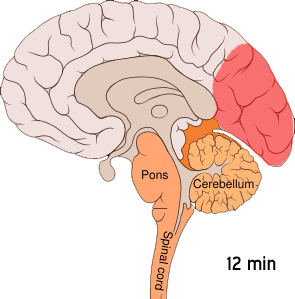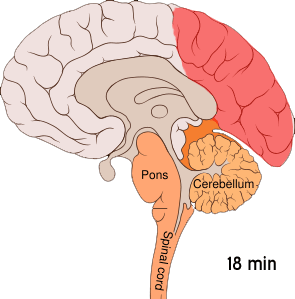
This wave of intense excitement followed by inactivity spreads across the visual cortex – corrupting visual information from the eyes and creating strange auras.
This phenomenon, known as cortical spreading depression (CSD), is responsible for the peculiar auras that slowly take over a migraineur’s visual field. When a neuron is affected by the spreading depression, it stops accurately processing visual information from the eyes – instead interpreting signals into disturbances like flashing lights or blurry blind spots.

Cortical spreading depression is not unique to the visual cortex; it can occur throughout the grey matter. The reason why this wave of electrical activity originates in the visual cortex is because this region of the brain is very densely crammed with neurons – increasing the risk of one neuron’s aberrant activity affecting the many cells around it.
CSD & Pain
The effects of CSD extend far beyond interfering with vision. Neuron excitation in the visual cortex activates adjacent trigeminal nerves which span your face from temple to jaw. These nerves run parallel to major blood vessels of the meninges (the cushioning tissue between your skull and brain) and, when activated, trigger the release of pain-generating substances into the bloodstream. As a result, the meninges become inflamed, causing blood vessels to widen and put further pressure on the tissue – intensifying the pain.
To make matters worse, these waves of neural activity up-regulate the expression of genes encoding pro-inflammatory molecules (such as COX-2, the target of many painkillers) exacerbating the sensation of pain. Additionally, CSD activates metalloproteinases, provoking the blood-brain barrier to leak; pro-inflammatory molecules, as well as the K+ and glutamate from earlier, are now free to flood into the major facial blood vessels. In combination, these molecules sensitise the nerves in the neck and jaw, causing the notorious aches and stiffness many migraine sufferers endure. This sensitisation is also why normally innocuous processes (like coughing and yawning) hurt and cause tension to build up during a migraine.
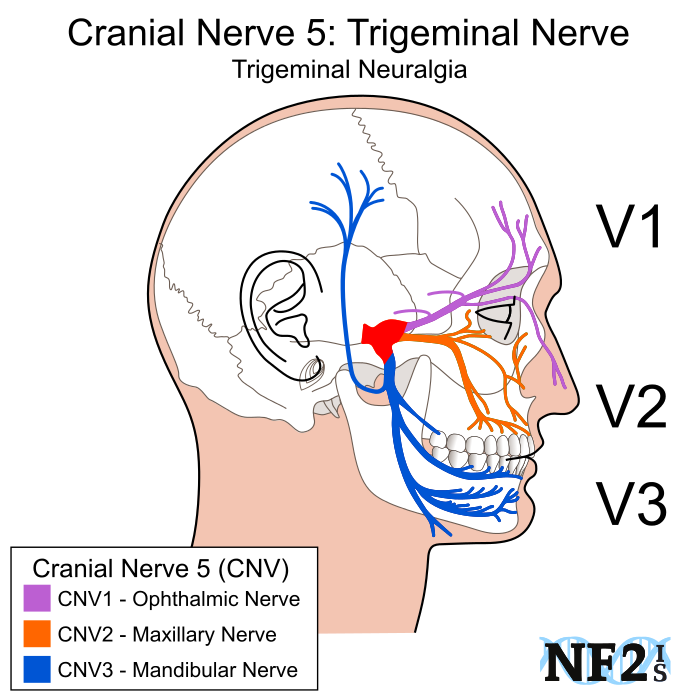
The spreading depression is first transmitted to the ophthalmic nerve branch and is then propagated to the mandibular nerves, bringing the pain along with it.
Potential Treatments
None of these excruciating effects are felt in ordinary headaches where CSD plays no role; to reiterate, the intense pain felt by migraine sufferers comes down to the unique biology of a migraine attack – it has nothing to do with low pain thresholds and its far more than just a bad headache.
Currently, the majority of migraine treatments either suppress pro-inflammatory molecule production or tackle the excessive blood vessel widening which puts pressure on the meninges. While they are useful in dealing with the headache aspect of a migraine attack, they have no effect on auras and fail to address the root cause of the migraine: the CSD.
Drugs that interfere with dopamine and serotonin pathways have been proven effective, quite possibly because they control neurotransmitter release and hence limit excitation of adjacent neurons in CSD – curbing the spread of depolarisation. Potential novel treatments also address the K+ ion channel dysfunction that triggers CSD, preventing both the aura and the pain that follows it.
When it comes to migraine treatment, the classic proverb rings true – prevention is better than cure. However, there are shockingly few effective migraine prophylactics.
Research into what triggers the K+ ion efflux of CSD in the first place and why sufferers are genetically predisposed to having migraines could spare the nearly 10 million of us in the UK from frequent bouts of agony.
.
Bibliography:
- Pietrobon, D., Striessnig, J. Neurobiology of migraine. Nat Rev Neurosci 4, 386–398 (2003). https://doi.org/10.1038/nrn1102
- Lauritzen, M. (1987). Cortical spreading depression as a putative migraine mechanism. Trends in Neurosciences, 10(1), 8–13. doi:10.1016/0166-2236(87)90115-9
- Charles, A. C., & Baca, S. M. (2013). Cortical spreading depression and migraine. Nature Reviews Neurology, 9(11), 637–644. doi:10.1038/nrneurol.2013.192
- Medscape.com. (2019). Migraine Headache: Practice Essentials, Background, Pathophysiology. [online] Available at: https://emedicine.medscape.com/article/1142556-overview#a3.
- Spierings, E. L. H. (2001). MECHANISM OF MIGRAINE AND ACTION OF ANTIMIGRAINE MEDICATIONS*. Medical Clinics of North America, 85(4), 943–958. doi:10.1016/s0025-7125(05)70352-7
I’ve written this post for Migraine Awareness Week – if you wish to support research into treatments for this debilitating neurological condition, consider donating to Brain Research UK.



































 One of the major toxic compounds present in bluebells are polyhydroxylated pyrrolidines (such as nectrisine) which are found in the viscous sap that seeps out of the plant’s nodding stems. These compounds are analogues of sugar but are actually potent glucosidase inhibitors. Due to its many -OH groups, the mammalian body mistakes nectrisine as a sugar and processes it as such – inadvertantly allowing the molecule to interfere with respiration.
One of the major toxic compounds present in bluebells are polyhydroxylated pyrrolidines (such as nectrisine) which are found in the viscous sap that seeps out of the plant’s nodding stems. These compounds are analogues of sugar but are actually potent glucosidase inhibitors. Due to its many -OH groups, the mammalian body mistakes nectrisine as a sugar and processes it as such – inadvertantly allowing the molecule to interfere with respiration.








