Cats Are Not Peas is a popular science book regarding the history of genetics and how the field evolved from early theories of fertilisation and trait inheritance to a modern understanding of gene expression and disorders. The common theme running through the book is the author, Laura Gould’s, quest to better understand her rare male calico cat, George.
Ginger cats are generally male and calico (aka. tortoiseshell) cats are almost never male. The allele for a ginger coat colour is recessive and the coat colour gene is carried on the X chromosome. Female cats have two X chromosomes, so, in order for a female to be ginger, she must have the ginger allele on both of her X chromosomes- which is unlikely in a typical feline gene pool. If she has only one ginger allele, she will usually be a tortoiseshell cat. At the sixty-four-cell stage of embryonic development, a form of dosage compensation known as X-inactivation randomly silences one X chromosome of the pair in each embryonic cell, independently of neighbouring cells. The silenced chromosome is maintained as a Barr body for all future cell divisions. So each cell has either an active ginger allele or an active black allele – resulting in a coat that is a mosaic of the two coat colours.
Since Cats Are Not Peas is written for a non-specialist audience, Gould does not delve into the details of Barr body formation. However, this piqued my interest and I researched X-inactivation further. Calico cats were actually involved in the history of X-inactivation study; in 1949, Murray Barr and his PhD student Ewart Bertram identified a dark, condensed structure (a Barr body) close to the nucleolus in the dissected motoneurons of a female calico cat. In 1961, Mary Lyon formulated her X-inactivation theory which proposed a process which leads to the global silencing of the entire X chromosome. This prompted further investigation into how embryonic cells can ‘count’ the number of X chromosomes to inactivate (always equal to (n-1) chromosomes). For instance, in an XX pair only one chromosome is silenced whereas in the case of triple X syndrome two X chromosomes are silenced. The blocking hypothesis suggests that ‘counting’ is achieved by small amounts of a blocking factor binding to the Xic locus of a single X chromosome in the pair. The other X chromosome(s) will then be inactivated as they are unblocked.

Another line of investigation regarded the ‘choice’ of which chromosome to inactivate; what were the molecular mechanisms of skewed choice or randomness? Non-random choice can be a result of secondary selection for/against certain embryonic cells – if an allele on the active chromosome leads to cell death, all surviving cells in the embryo will have that X chromosome inactivated. Alternatively, primary skewing of the X inactivation choice may be a result of certain X chromosomes being more resistant to inactivation. A key example of this is the X-controlling element (Xce) found in mice. In 1972, Bruce Cattanach identified that resistance to X-inactivation is influenced by which Xce allele each chromosome had.
Returning to the book, calico cats are not simply black and orange; the majority also have white markings which are a result of autosomal influence. Here Gould explains the concept of gene expression masking; the autosomal allele for piebalding is expressed and masks the coat colour alleles by giving the skin and coat an unpigmented appearance.
If a male cat inherits one ginger allele, he will certainly have a ginger coloured coat. Since they only have one X chromosome, male cats are almost never tortoiseshell as there isn’t a competing dominant allele. Very occasionally, male tortoiseshell cats crop up; generally, they have two X chromosomes (with only one containing the ginger allele) and a Y chromosome as a result of chromosomal non-disjunction.
Again, I did some further research into mechanisms of nondisjunction in Klinefelter’s Syndrome. The nondisjunction event may occur in paternal meiosis I or either of the maternal meioses. Nondisjunction occurs when there is inactivation of topoisomerase II, condensin, or separase which results in both homologous chromosomes or sister chromatids being pulled to the same pole of the cell during anaphase. The case with George is even more peculiar, however, since this calico cat not only has Klinefelter’s syndrome (as a result of the XXY sex chromosomes) but also mosaicism as a result of chromosomal nondisjunction during embryonic mitosis – some of his cells contain three sex chromosomes whereas some only contain the typical XY pair.
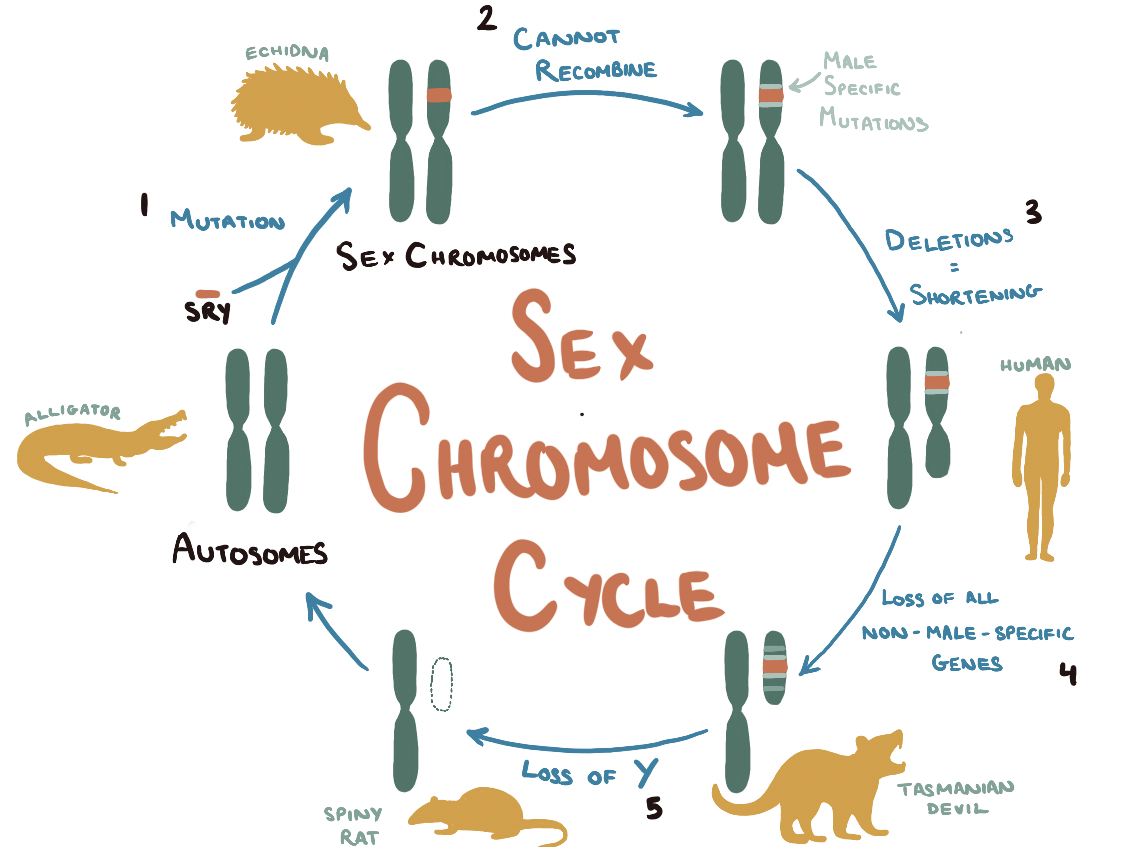
Cats Are Not Peas takes many tangents to explore other topics related to sex chromosomes. Gould starts an interesting discussion into the size disparity between the X and Y chromosome however, since she wrote the book in 1996, newer studies have elucidated the mechanisms by which genetic information is gradually lost from the Y chromosome (on an evolutionary timescale).
Prior to the evolution of mammals, sex determination did not follow a chromosomal blueprint. Rather it was decided by environmental factors. For example, alligators’ sex entirely depends on incubation and egg temperature at the time of hatching. As a result, every one of their chromosomes belongs to a matching set as they lack distinctive sex chromosomes. At some point, very early in the evolution of mammals, an ancient form of the SOX3 gene, involved in brain development pathways, randomly mutated to form the SRY on a single autosome. The SRY gene codes for the testis-determining transcription factor (TDF) which binds to DNA to start a cascade of biochemical reactions leading to the differentiation of sexless primordial gonad cells into the sperm-producing cells of the testes.

The switch from SOX3 to SRY was the first step in a long path of mutations, a growing divide between the SRY-containing autosome and its counterpart—culminating in two very different sex chromosomes. From comparing our genomes to those of early mammals, such as marsupials, geneticists have identified that the modern Y chromosome shares just four genes with its much larger autosomal ancestor. Due to the sex chromosomes inability to undergo recombination, the Y chromosome has gradually acquired a slew of deleterious mutations—filling the chromosome with ‘junk’ DNA and shortening it through deletion mutations. As the Y chromosome mutates, it becomes less able to recombine with its X counterpart and continues to lose genes resulting in a vicious cycle of shrinkage.
Dasyurid marsupials, such as the Tasmanian devil, have a Y that has been whittled down to just 10 megabases in length—a little genetic fragment containing just SRY and a few sperm-producing genes. This degradation is taken to the extreme in bandicoots. These marsupials use their miniscule Y chromosome (holding nothing but SRY, the last in a long line of seemingly disposable genes) for sex-differentiation and gonad formation, before eliminating it entirely from all other embryonic cells. Such a small chromosome, essentially a single gene, is at great risk of further loss.
Only two species have ventured beyond this point in chromosomal evolution: the ryukyu spiny rat and the transcaucasian mole vole. Their Y chromosome has diminished to the point where it has been taken up by its X counterpart through the process of translocation. Now instead of XX females and XY males, the sex-determination system is XX females and XX’ males (where X’ is an identical X chromosome with an addition of the SRY gene).
The X’ will not be able to entirely recombine with the X and gradually it will accumulate mutations—diverging in function and shortening to produce a new Y chromosome. In this way, the same Y chromosome evolution described earlier can renew itself cyclically in a sex chromosome cycle.
Cats Are Not Peas is a great read which explains many of the basic genetics concepts learnt in Year 1 of the MBiochem course such as X-inactivation, masking, variable gene expression, polygenes, mosaicism and chimerism. The book doesn’t include many recent developments in genetics (such as sequencing technologies) as it was written pre-Human Genome Project in 1996. However, it still gives an interesting perspective on genetics research and the history of the field up until that point. It also piqued my interest in X inactivation and Y chromosome shortening and served as a starting point for further research.
References:
Gould, L. (1996). Cats are not Peas. New York, Ny Springer New York.
Avner, P. and Heard, E. (2001). X-chromosome inactivation: counting, choice and initiation. Nature Reviews Genetics, 2(1), pp.59–67.
Morey C, Avner P (2011) The Demoiselle of X-Inactivation: 50 Years Old and As Trendy and Mesmerising As Ever.
PLOS Genetics 7(7): e1002212. https://doi.org/10.1371/journal.pgen.1002212
Kashimada, K. and P. Koopman. “Sry: the master switch in mammalian sex determination.” Development 137 (2010): 3921 – 3930.
HHMI BioInteractive. (n.d.). Evolution of the Y Chromosome. [online] Available at: https://www.biointeractive.org/classroom-resources/evolution-y-chromosome [Accessed 14 September 2021].
Matveevsky, Sergey et al. “Chromosomal Evolution in Mole Voles Ellobius (Cricetidae, Rodentia): Bizarre Sex Chromosomes, Variable Autosomes and Meiosis.” Genes vol. 8,11 306. 3 Nov. 2017, doi:10.3390/genes8110306
Benjamin L S Furman, David C H Metzger, Iulia Darolti, Alison E Wright, Benjamin A Sandkam, Pedro Almeida, Jacelyn J Shu, Judith E Mank, Sex Chromosome Evolution: So Many Exceptions to the Rules, Genome Biology and Evolution, Volume 12, Issue 6, June 2020, Pages 750–763, https://doi.org/10.1093/gbe/evaa081
















































 One of the major toxic compounds present in bluebells are polyhydroxylated pyrrolidines (such as nectrisine) which are found in the viscous sap that seeps out of the plant’s nodding stems. These compounds are analogues of sugar but are actually potent glucosidase inhibitors. Due to its many -OH groups, the mammalian body mistakes nectrisine as a sugar and processes it as such – inadvertantly allowing the molecule to interfere with respiration.
One of the major toxic compounds present in bluebells are polyhydroxylated pyrrolidines (such as nectrisine) which are found in the viscous sap that seeps out of the plant’s nodding stems. These compounds are analogues of sugar but are actually potent glucosidase inhibitors. Due to its many -OH groups, the mammalian body mistakes nectrisine as a sugar and processes it as such – inadvertantly allowing the molecule to interfere with respiration.



