Before the first cells of our bodies are even formed, we all owe our lives to electricity. It plays a crucial role in the first and most important race of our lives, regardless of whether we grow up to be marathon runners. This is the race of fertilisation, in which the finish line is an egg cell and the winner is the sperm cell that made each one of us.

In the same way that professional athletes have light, aerodynamic bodies and strong legs to propel them, sperm cells are streamlined and specialised to win their race. But instead of powering their movement with muscular limbs, sperm cells have powerful tails, known as flagella, which beat from side to side, tunnelling their way through the viscous fluid of the female reproductive tract.
At first, the race is akin to a marathon in which covering the distance between the vaginal opening and the uterus, without running out of energy, is the main priority. Dyenins, molecular motors embedded in the base of the flagella, produce a rapid symmetrical bending movement which quickly move the sperm cells to the upper reproductive tract in under half an hour.
From Ions to Energy
Just like the motors which keep the blades of ceiling fans spinning, these molecular motors need a constant supply of energy to keep the flagella beating. This energy comes in the form of tiny molecules called ATP which are continuously pumped out of miniature factories known as mitochondria. Mitochondria are bean-shaped organelles which have two membranes – an outer membrane which covers the organelle like a skin and a highly-folded inner membrane keeps important chemicals inside the organelle. There are mitochondria in all the cells in our bodies, but sperm cells need so much ATP to swim all the way to the egg that they are absolutely brimming with them. To produce all that ATP, mitochondria themselves require electrical energy.
So how do they get this electrical energy? First, let’s quickly return to our analogy of the ceiling fan, which uses electrical energy directly. Flick the switch on and extremely tiny charged particles flow through the wires of the fan motor. Electricity is really just the flow of any charged particle; when electricity flows, energy transfers along with it. The device you are reading this on is powered by the flow of negatively charged electrons. Our bodies, on the other hand, are mainly driven by slightly larger, positively charged particles: ions.
Our mitochondria are extra special because they can make use of both ions and electrons. First, these miniature factories shuttle around electrons to create flows of charge: bioelectricity. The resulting energy transfer is used to push positively charged hydrogen ions (H+) out of the mitochondrial inner membrane into the empty space between membranes. This causes a build-up of H+ ions on one side of the inner membrane and a loss of H+ ions on the other side. This results in one side of the inner membrane being more positively charged than the other – creating an electrical potential gradient (aka a voltage).
As I write this article, my abandoned cup of piping-hot tea is quickly turning cold; in the same way that heat tends to flow from a hot object to the cold surrounding air, charged particles tend to move from a positively charged area to a negatively charged one, down to the electric potential gradient. H+ ions in the mitochondria’s intermembrane space try to squeeze back through the inner membrane to the more negatively charged side.
But biological membranes repel H+ ions because they are charged so the ions cannot pass through freely. Mitochondria take advantage of that fact by having lots of specialised ion channels embedded in their inner membrane; these channels allow H+ ions to easily diffuse through to the more negatively charged side. But there is a catch. As H+ ions flow through the specialised channels, their electrical potential energy is transferred to the channel and used to manufacture more ATP!
We have now seen two ways sperm use bioelectricity, in their mitochondria, to help spin their flagella. Bioelectricity is essential for the repetitive side-to-side beating needed to get the sperm to the uterus. But the sperm cell’s journey doesn’t end there…

A Sticky Situation
When a sperm cell draws nearer to the egg cell, its marathon transforms into an obstacle course. Before it can even begin to navigate the sharp turns and swooping bends of the fallopian tubes, the sperm gets trapped by spindly finger-like projections at the tube entrance.
To free itself from the clutches of the upper reproductive tract, a change of movement is required. Sperm cells don’t have another type of molecular motor which takes over the flagella, but they do have a trick up their metaphorical sleeves, and this too involves bioelectricity.

As the sperm cell travels through the uterus, the environment changes. Earlier, the cell journeyed through a mixture of seminal and cervical fluid. Now, as it reaches the fallopian tube, it must swim through the sticky fluid released by the ovaries. Known as follicular fluid, this stuff is far richer in positively charged sodium (Na+) ions than the sperm cell’s cytoplasm. As a result, there’ is an imbalance of charge between the inside and outside of the sperm cell: a voltage .
This voltage triggers the opening of specialised exchanger proteins in the sperm cell membrane which can trade sodium ions outside the cell for hydrogen ions within the cell. As the sperm cell pumps out H+ ions through this exchanger, the concentration of H+ ions rapidly plummets. The pH of the cell’s cytoplasm is inversely proportional to H+ concentration; as the ion concentrations decreases, pH increases – making the cytoplasm more alkaline. This is crucial for initiating the next phase of sperm movement.
Modifying Movement

While the sperm cell is still held hostage by the spindly projections of the fallopian tube, it gets bathed in even more follicular fluid, which is rich in the hormone progesterone. Sperm cells are highly receptive to even a trillionth of an increase in progesterone concentration, so this flood of hormone induces great changes in its behaviour.
In combination with the alkaline environment, progesterone triggers the opening of CatSper channels (Cationic channels of Sperm) which allow positively charged calcium ions to flood into the sperm, yet again modifying the voltage across the sperm cell membrane.
More importantly, these calcium ions influence the dyenins in sperm flagella, so they produce an asymmetrical, whip-like movement. Adding calcium ions is akin to adding gears between the motor and the wheels of a car when you need more power to drive up a steep hill. The Ca2+ ions slow down the molecular motors and increases the overall bending of the flagella so that each beat of the sperm’s tail is more forceful. With this extra power, sperm cells can kick away from the clutches of the fallopian tube entrance. The asymmetric movement, combined with the sperm cell’s attraction to progesterone, allow it to steer a course towards the egg cell.

Bioelectricity, in the form of changing voltages and positive charge flow, is vital for sperm cells to break free from the microvilli projections at the entrance and make that final push through the fallopian tubes to the finish line of fertilisation. Staggeringly few sperm cells make it. Only one sperm cell out of every million that enter the female reproductive tract even makes it to the fallopian tube and less than a hundred ever come close to the egg. To put this in context, a healthy man can ejaculate about a teaspoon of semen, teeming with 500 million sperm cells – each sperm has a shockingly slim 0.00002% chance of even meeting the egg.
Without the powerful whipping motion that this bioelectricity enables, sperm cells can’t fertilise an egg cell and so a male cannot transfer genetic material to produce offspring. Ultimately, bioelectricity issues can result in infertility, a condition that affects 7 in 10 British couples.
Polyspermery: It’s A Draw
The converse issue, wherein lots of sperm cells are very efficient swimmers and reach the egg cell at the exact same time, also hinders reproduction. In a phenomenon known as polyspermy, two or more sperm cells fertilise the egg cell at the same. In terms of our race metaphor, polyspermy happens when two competing sperm cells ‘draw’ and there is no winner.
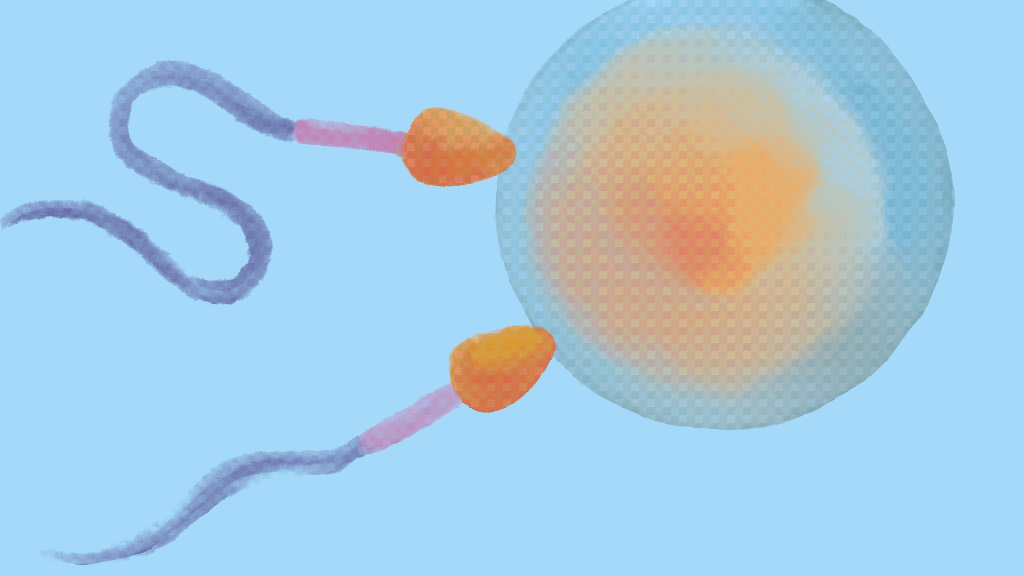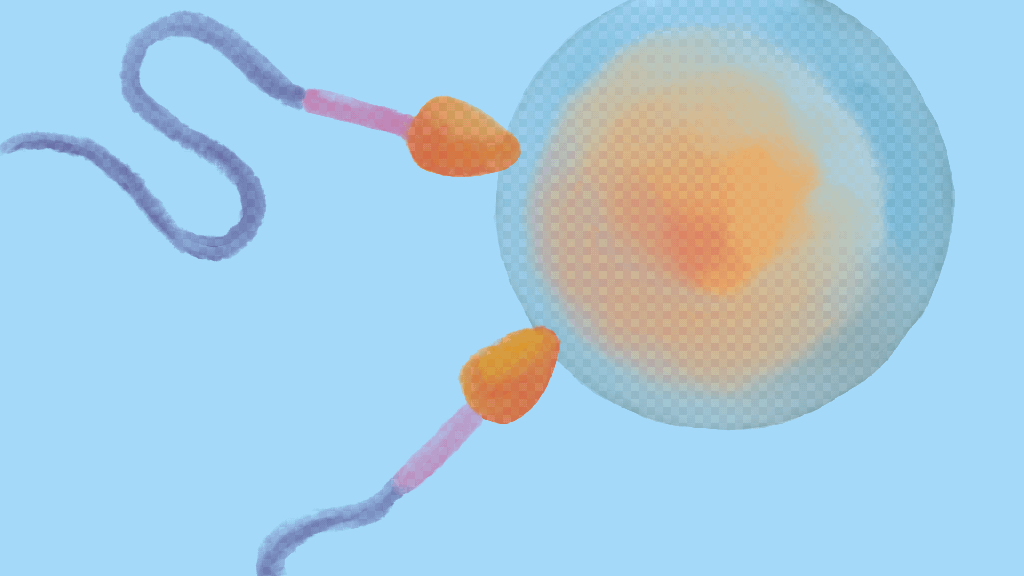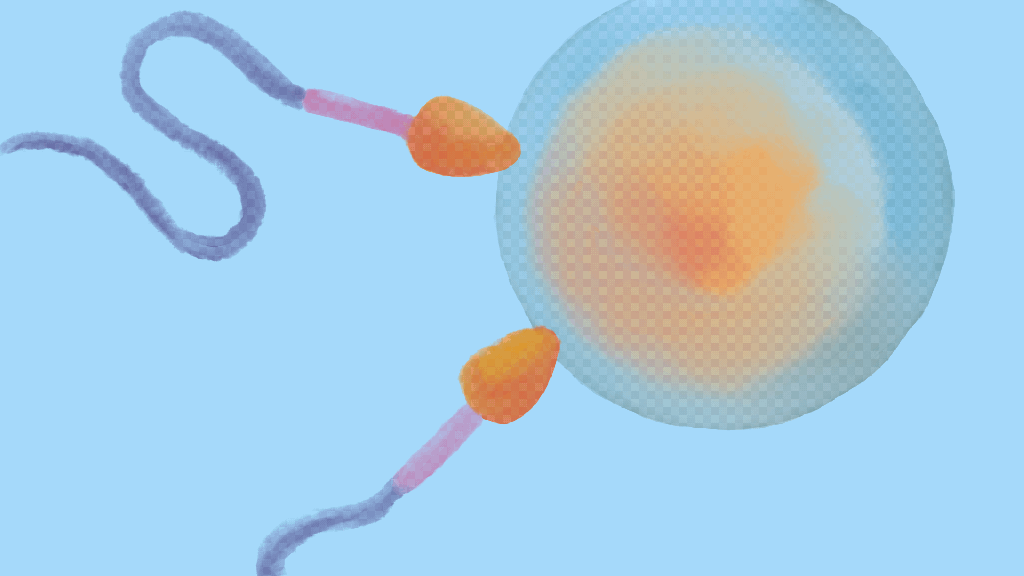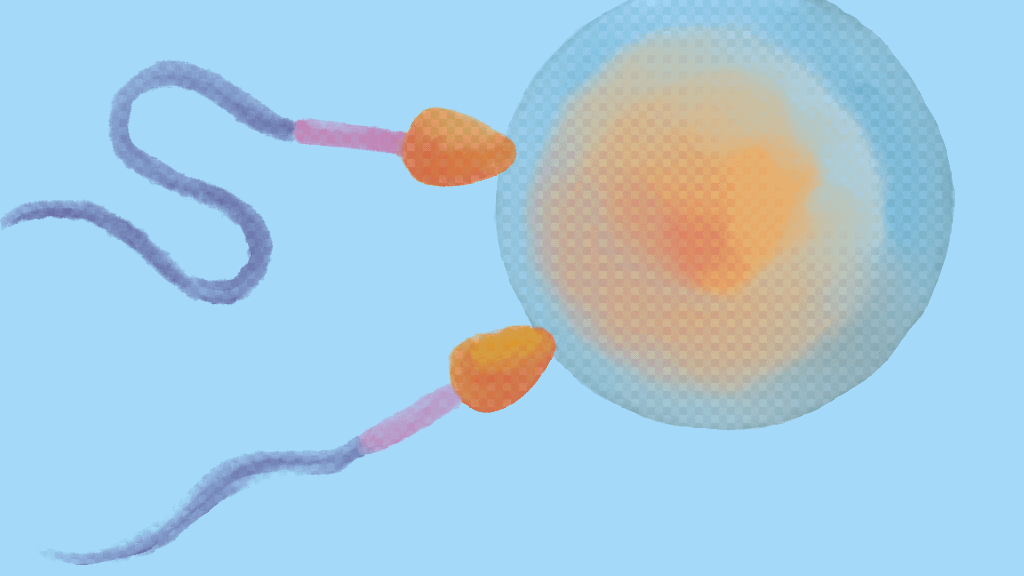
An egg cell fertilised by two or more sperm cells cannot divide the contributed genes evenly to split into new cells and produce an embryo, so the fertilised cell dies. As we’ll see shortly, polyspermy isn’t a major threat for humans since multiple sperm cells rarely reach the egg at the exact same instant. However, the risk of polyspermy is a serious issue for many marine animals that rely on external fertilisation to form offspring.
Sea Urchin Sex
Consider sea urchins, probably the spikiest creatures beneath the waves. Male and female sea urchins reproduce by releasing clouds of sperm and egg cells into the water and hoping they fuse to form offspring larvae. Compared to humans, many more sea urchin sperm cells approach a single egg simultaneously. To prevent polyspermy, a fertilised egg cell needs to act fast and block access to any more competitor sperm. This calls for bioelectricity.
An urchin egg cell waiting to be fertilised contains fewer positively charged ions than the surrounding seawater. This disparity in positive charge between its internal and external environment create a voltage known as a membrane potential; in the case of an unfertilised sea urchin egg cell, this membrane potential is -70 mV.
The very instant that the first sperm cell, the winner of the fertilisation race, binds to the egg cell, it triggers calcium and sodium ion channels to open and allow the positively charged ions to flood in. This results in a reversal of membrane potential as the inside of the egg cell becomes more positive than its surroundings, depolarising the membrane to +25 mV.
This rapid switch in membrane charge inhibits additional sperm cells from binding, however the exact mechanism remains a mystery. One prevailing theory is that a positive membrane potential inactivates the specialised slots in the egg cell membrane that allow sperm cells to dock in – preventing additional sperm from fusing with an already fertilised egg.
Alternatively, the positively charged proteins on the tip of the sperm cell, which fuse it to an egg, might be repelled by the newly positive membrane of the egg cell, ultimately inhibiting fusion and polyspermy. Regardless of the true mechanism, it is evident that bioelectricity plays an important role in guaranteeing successful fertilisation, and not only in sea urchins.
Slow Blocks
Polyspermy is not as immediate a threat to fertilisation for humans due to the relatively low sperm-to-egg ratio and the complexities of navigating the female reproductive tract. Instead of a fast, electrical block, our fertilised egg cells block out additional sperm through chemical methods: a slow block . To understand how human egg cells do this, we need to first look at its structure and its vulnerability to polyspermy.
Like all body cells, egg cells have a standard membrane that keeps all its cellular machinery and DNA inside, safe and secure. On top of this, egg cells are coated in a transparent jelly layer called the zona pellucida, which allows sperm cells to tether to the egg during fertilisation. The slow block to polyspermy involves changes in both the inner membrane and the zona pellucida.
Within minutes of fertilisation, the ‘winning’ sperm triggers calcium ions from within the egg cell to be released from their storage spaces and rush through the cell membrane. But unlike in the sea urchin eggs, this ion movement is not a one-time event for human egg cells. In the next minute, another wave of positively charged ions are sent out, and this whole process is repeated over and over and over. The end result is the formation of a fluctuating membrane potential which steadily flicks from positive to negative like a metronome.
These calcium ion oscillations themselves do not deter additional sperm. Instead, they orchestrate a cascade of reactions which trigger little pockets of enzymes to be pushed out of the egg cell. The enzymes inside can then dissolve the bonds between the membrane and the zona pellucida, pushing away the jelly layer and all the other attached competitor sperm cells along with it.
An IVF Issue
For natural human conception, this slow block is sufficient. However, within the last fifty years, this process has been unable to keep up with a novel form of reproduction we have pioneered: in vitro fertilisation (IVF). The conditions of IVF are very different to that of natural conception – the reproductive tract with its pH gradient, microvilli obstacles and sharp bend is swapped out for a simple laboratory dish. Many more sperm cell are incubated with a single egg to increase the chance of successful fertilisation. The consequence is that, when the playing field for sperm is levelled to this extent, the classic slow block might not act fast enough to block out additional sperm cells. Up to 10% of fertilised eggs in IVF become non-viable due to polyspermy.
Recent studies suggest that egg cell quality could influence polyspermy block speed. By studying our rodent friends, biochemists have found that older mice eggs are more likely to have abnormal calcium signalling. The oscillations are irregular, like a broken metronome swaying wildly out of time. As a result, not enough enzyme pockets are recruited to the membrane. This means that the zona pellucida does not completely detach from the egg cell – allowing some sperm cells to stay stuck in place and squeeze inside.
Moreover, the in vitro setting and egg freezing involved in IVF decrease both the frequency and duration of calcium ion oscillations. Whilst screening eggs for their quality is still out of reach, bioelectricity research could improve egg preservation techniques – bringing the finish line closer in the most important race of our lives.