Through The Looking Glass
Lewis Carroll’s sequel to Alice’s Adventures in Wonderland was a firm favourite of mine in childhood. After all these years, memories of the mirror world Alice falls into come flooding back to me as I study organic chemistry.
In Through the Looking Glass, initially the mirror-world Alice explores is almost exactly the same as the world we are all familiar with except it’s a mirror image. For instance, Alice opens a book of poems and expects to see the words

but is astounded to instead find written

The words on her page are mirror images of each other but, within these words, some of the letters appear identical to what Alice expected to find (A, W, & O). An ‘A’ from the mirror-world is a superimposable mirror-image of an ‘A’ from the regular world. For some letters, however, this is certainly not the case; J, B, E, R, C, and K are not identical to their mirror-world counterparts. These letters are non-superimposable mirror images.
Now let’s imagine an even stranger world where the word ‘JABBERWOCKY’ is arranged like this:

In this three-dimensional arrangement, these little groups of letters are bonded together and frozen in this specific spatial arrangement with ‘C’ protruding out of the page and ‘KY’ extending back into the page.
In a mirror-world, we would expect to find the mirror image of this strange 3-D word:

These two 3-D words are mirror images of each other but they are non-superimposable. Even if Alice were to reach into the page and twist the ‘mirror-world’ word around by 180 degrees, it would not be identical to our original 3-D word since the ‘C’ would be extending back into the page instead of protruding out of it.

mirror-world rotation

original
instead of
Hence, the original 3-D word and the mirror-world word are non-superimposable mirror images. This Lewis Carroll analogy has been stretched to its limits so let’s return the realm of organic chemistry…
Many organic compounds also have non-superimposable mirror-image molecules. Such pairs of molecule are known as enantiomers. These doppelgangers are optical isomers since they differ in terms of spatial arrangement and not atom connectivity. Optical isomerism can arise from the fascinating concept of chirality. Chiral molecules possess at least one carbon atom bonded to four different groups (a chiral centre) and are not identical to their mirror image molecule.
Any molecule which exhibits reflective symmetry is, by definition, achiral; this means that all the amazing asymmetrical molecules that make up biological systems are chiral – opening up new avenues of complexity and complications.
When chiral molecules are synthesised in the laboratory, the specific enantiomer formed is determined by random chance and so chemists usually end up with a 50/50 split of the two enantiomers; this mixture, known as a racemate, is chemically pure but has different optical properties and different interactions in biological systems – ushering in a plethora of unforeseen obstacles and opportunities.
Drugs with Doppelgangers
All the components of living systems, right down to enzyme and receptor molecules, are chiral and so can only interact with one of the enantiomers of any pair of molecular twins. Therefore, the ‘active’ enantiomer of a drug has a three-dimensional structure that perfectly aligns with the binding site whereas the inactive enantiomer cannot bind in the same way no matter how much you rotate it.
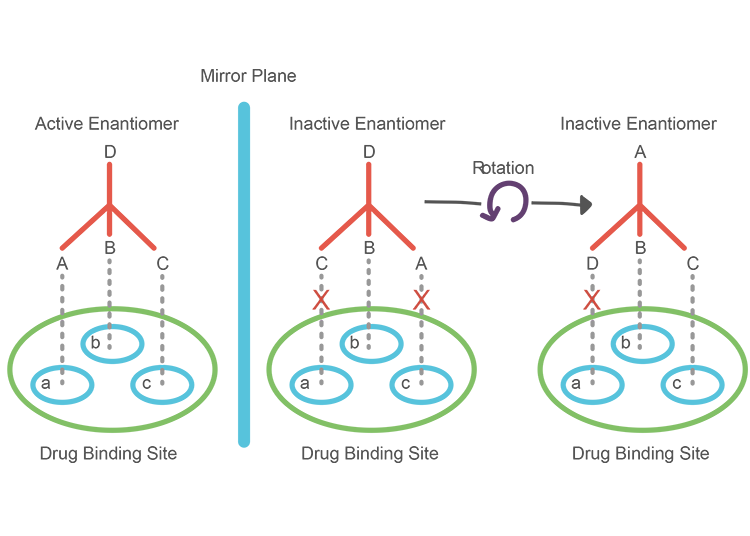
A great example of this is epinephrine, more commonly known as adrenaline; the receptor site on the post-synaptic membrane of a neuron contains three binding sites complementary to the three functional groups of the more active enantiomer. Firstly, there is an anionic site which can form electrostatic forces of attraction with the positively charged ammonium ion group. At the opposite end of the molecule, the phenolic ring is attracted to the flat site of the receptor with π-π interactions.
The final receptor site can form hydogen bonds with the β-hydroxyl group attached to the chiral carbon in the active enantiomer of epinephrine. The inactive enantiomer, on the other hand, has a different orientation of the β-hydroxyl group so cannot form this crucial intermolecular bond. Hence, this enantiomer only has two points of contact with the receptor molecule – resulting in a hundred-fold decrease in activity which makes it ‘inactive’.
Each molecule in a synthetic epinephrine racemate has a 50/50 chance of being the active enantiomer and, thus, an effective neurotransmitter which means that higher doses are needed to achieve a desirable outcome such as an increase in post-synaptic action potentials. Needless to say, organisms would waste far too much energy synthesising biological molecules if only half of them could be metabolically active. Otherwise, for both enantiomers to be catalysed, our DNA would need to code for twice as many enzymes and receptor proteins which is similarly inefficient. In order to overcome this issue of ensuring complementary chirality, life plays by an entirely different set of rules.
The Principle of Homochirality

All life forms on earth exclusively synthesise and use L-amino acids and D-sugars so all naturally-occurring molecules are enantiomerically pure. This principle of homochirality can be explained from a biological standpoint by turning back the hands of time to the point where life first starting flourishing on earth.
When early life was first evolving, the enzymes synthesised by a microorganism would have been complementary to either the L-enantiomer or D-enantiomer, however the microorganism’s environment would have consisted mainly of one enatiomer or the other. The only microorganisms that survived and multiplied in our early environment would be ones that produces enzymes complementary to the most prevalent enantiomer of any molecule. Since early microorganisms were in environments abundant in D-sugars and L-amino acids, selective pressures would give rise to populations of microorganisms with enzymes and receptors only complementary to these enantiomers. Hence, every subsequent organism evolved from these early life forms would also be force to use the same amino acid and sugar enantiomers as its predecessors: resulting in universal enantiomer preferences.

There is a straightforward reason as to why D-sugars are more abundant than their L-enantiomer counterparts; sugars can spontaneously undergo cyclisation reactions to form the ring structures we are more familiar with. D-sugars form more stable rings than L-sugars and so are more prevalent in our environment.

circularly polarised light
The reason for the prevalence of L-amino acid is less intuitive and requires us to venture even further back in time to before life even began. Chirality doesn’t just exist at a molecular level; our galaxy itself rotates with a chiral spin which causes cosmic dust to circularly polarise the light travelling through space. From studying amino acid residue in meteors and comets, we can ascertain that circularly polarised light degrades D-amino acids to a greater extent than L-amino acids, resulting in the greater stability of L-amino acids.
This discrepancy in amino acid degradation, however, is not sufficient to explain the exclusive presence of L-amino acids in biological systems. Hence, we must consider the biochemistry behind the formation of chiral compounds.
In a primordial mixture of amino acids on earth, L-enantiomers act as auto-catalysts and catalyse their own formation, increasing the rate of L-enantiomer formation with increasing concentrations. Additionally, L-enantiomers can bind to D-enantiomers in a process known as mutual antagonism which inhibits auto-catalysis and further formation of the D-enantiomer. Auto-catalysis of L-amino acids, in combination with mutual antagonism, serve to exacerbate the discrepancy in amino acid ‘survival’, contributing to the favouring of L-amino acids over D-amino acids on our planet.
Isomer Inversion

Although almost all receptor and enzyme active sites in the body are only complementary to L-amino acids and D-sugars, certain enzymes, known as racemases, can convert a molecule’s inactive twin into the appropriate enantiomer in the process of stereochemical inversion. AMACR, for instance, converts a range of molecules from the R-enantiomer to the S-enantiomer.
One such molecule is the nation’s favourite painkiller: ibuprofen. Ibuprofen is administered as a racemate but undergoes a series of changes in the body (catalysed by AMACR) which convert all the R-ibuprofen to S-Ibuprofen which can inhibit cyclooxygenases and reduce the production of prostaglandins – hence creating an anti-inflammatory effect.
Increase in AMACR activity above certain threshold is hallmark of prostate cancer as AMACR is used to invert the chirality of molecules involved in accelerated cell proliferation and tumour growth. A prospective treatment of prostate cancer involves administering ibuprofen to prostate cells so that excess AMACR will catalyse the formation of S-Ibuprofen instead of molecules associated with tumour growth, resulting in a chemopreventive effect.
Mirror Milk
‘How would you like to live in Looking-glass House, Kitty?
I wonder if they’d give you milk in there?
Perhaps Looking-glass milk isn’t good to drink…’
In Through the Looking Glass, Alice raises the concern of her cat drinking looking-glass milk. From what we now understand of chirality, it is safe to say that neither the cat, nor Alice herself, would fare well in a mirror-world where their digestive enzymes cannot break down milk which is rich in D-amino acids an L-sugars (although the fats in milk, which are all achiral and therefore unchanged, could provide them with enough energy to make a quick escape).
But perhaps Kitty’s enantiomeric twin produces enzyme and receptor molecules proficient in synthesising and utilising these strange enantiomers.
Perhaps mirror-cats are partial to mirror-milk

Bibliography:
- Clegg, B. (2020). Ibuprofen. ChemistryWorld (RSC). 20 Mar. [Accessed 27 Mar. 2020].
- Brown, M. (2011). Ibuprofen: anticancer drug. [online] Chemistry World. Available at: https://www.chemistryworld.com/news/ibuprofen-anticancer-drug/3002034.article [Accessed 30 Mar. 2020].
- Wellford, S. (2016). Biological Homochirality: One of Life’s Greatest Mysteries. [online] Medium. Available at: https://medium.com/a-spoonful-of-sugar/biological-homochirality-one-of-lifes-greatest-mysteries-2031f4700c4b [Accessed 25 Mar 2020].
- Morozov, L. (1979). Mirror symmetry breaking in biochemical evolution. Origins of Life, 9(3), pp.187–217. [Accessed 30 Mar. 2020].

 Within the body, Fe (II) ions are best known for forming heme-complexes, porphyrin molecules which can be incorporated into proteins to create oxygen carriers (such as hemoglobin and myoglobin). Iron is not the only transition metal that can act as an oxygen carrier; copper is the central ion in the heme complexes of horseshoe crabs and snails – giving their blood a beautiful shade of blue.
Within the body, Fe (II) ions are best known for forming heme-complexes, porphyrin molecules which can be incorporated into proteins to create oxygen carriers (such as hemoglobin and myoglobin). Iron is not the only transition metal that can act as an oxygen carrier; copper is the central ion in the heme complexes of horseshoe crabs and snails – giving their blood a beautiful shade of blue.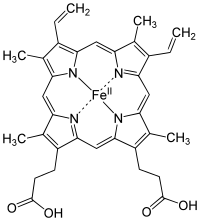




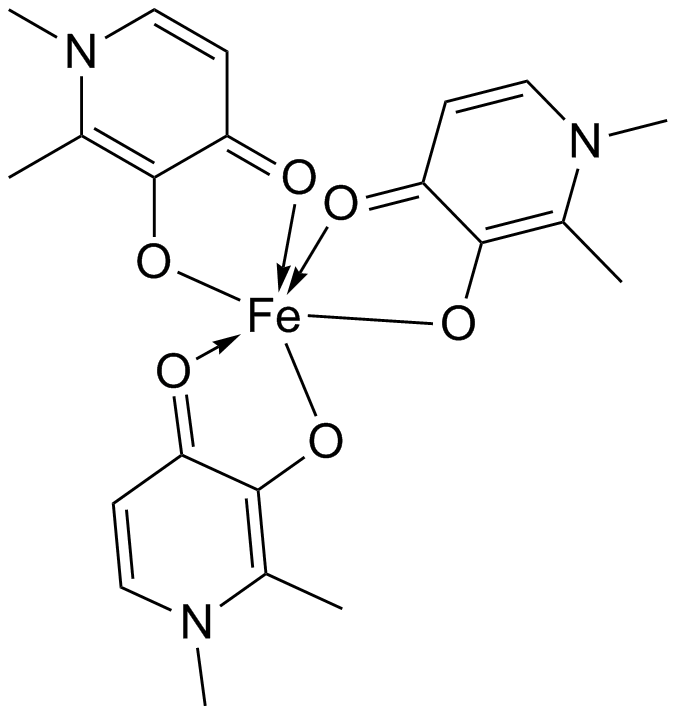 Iron chelators, such as deferiprone, can act as bidentate ligands and bind to excess rogue Fe (II) ions. This prevents the metal ions interfering with cellular respiration and aggregating amyloid precursor protein so no radical oxygen species are generated and plaque formation is prevented.
Iron chelators, such as deferiprone, can act as bidentate ligands and bind to excess rogue Fe (II) ions. This prevents the metal ions interfering with cellular respiration and aggregating amyloid precursor protein so no radical oxygen species are generated and plaque formation is prevented.